Everybody knows that metalsconduct electricity. If you apply a voltage, you get a current. Mostelements are metals. However the are two other classes of materials –semiconductors and insulators. Which class a material falls intodepends on how free the electrons are to move. In a metal many of theelectrons are completely free. In the diagrams below, the electronscan be considered to occupied the green shaded regions, and theregions in which electrons can have enough energy to be able to moveare coloured blue. This does not mean there are any electrons in theblue region. For there to be electrons free to move the blue andgreen regions have to overlap.
They do overlap for metals,hence metals can conduct electricity – the typical resistivity of ametal is![]() They don't overlap for semiconductors. Some energy may be given to theelectrons so they can move from the valence band, where they normally are, to the conduction band, where they may conduct electricity. This energy may be in the form of heat or light. This implies that if you heat a semiconductor, it's resistance falls, since it can conduct electricity better. The typical resistivity of a semiconductor is
They don't overlap for semiconductors. Some energy may be given to theelectrons so they can move from the valence band, where they normally are, to the conduction band, where they may conduct electricity. This energy may be in the form of heat or light. This implies that if you heat a semiconductor, it's resistance falls, since it can conduct electricity better. The typical resistivity of a semiconductor is![]()
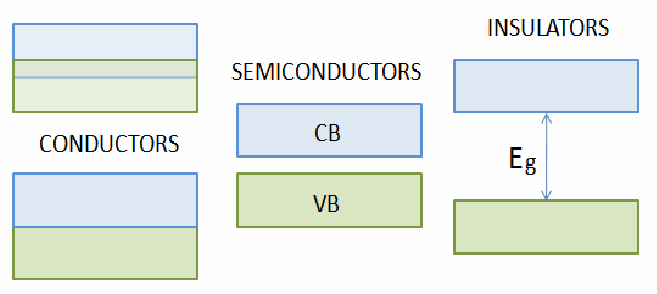
Forinsulators there is a much larger energy gap between the conductionand valence bands. The resistance of insulators is very high. A lotof energy must be given to the electrons to push them into theconduction band. The typical resistivity of an insulator is![]()
