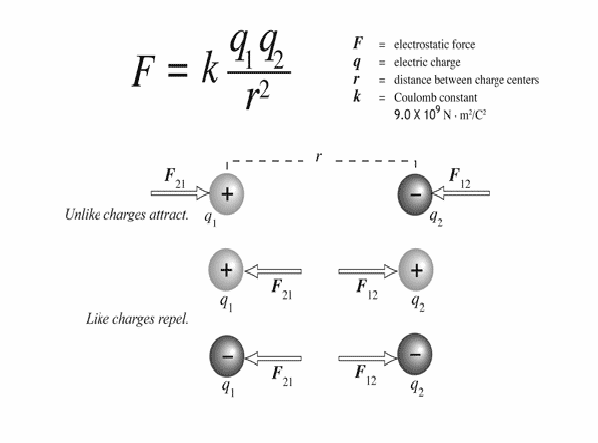
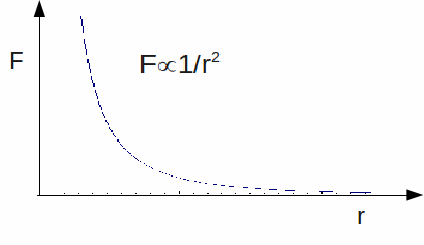If electric charges approach each other they exert forces on each other. There are two types of charge – positive and negative, and the force between them is repulsive or attractive according to the rules:
Like forces repel, opposite forces attract.
This means that two positive forces repel each other, two negative forces repel each other and positive forces are attracted to negative forces and vice versa.
The force always acts along the line of force joining the two charges, and the forces constitute a Newton's third law force pair, so they are equal in magnitude and opposite in direction.
All of these are expressed by Coulomb's Law, illustrated below.
If two or more charges are brought close together, the net force on each charge is the sum of the force due to all the other charges. Force is a vector, and each force will act in a particular direction. We can add these forces to obtain the net charge.
Coulomb's Law is an inverse square law, so the electric force decreases rapidly with increasing distance.