Whenever a nuclear reaction – fusion or fission releases energy – the products are in a lower energy state than the reactants. The source of this energy is the mass of the reactants, some of which is changed into energy via the equation![]()
If a light nucleus were totally separated into protons and neutrons, then the mass would be greater by a mass![]() (the binding energy) which is equivalent to a mass. Energy needs to be given to the nucleons to enable them to escape the nucleus.
(the binding energy) which is equivalent to a mass. Energy needs to be given to the nucleons to enable them to escape the nucleus.
Conversely, if a heavy nucleus is separated into protons and neutrons, then the mass is less by a mass![]() Energy is given out typically when a large nucleus splits into smaller nuclei.
Energy is given out typically when a large nucleus splits into smaller nuclei.
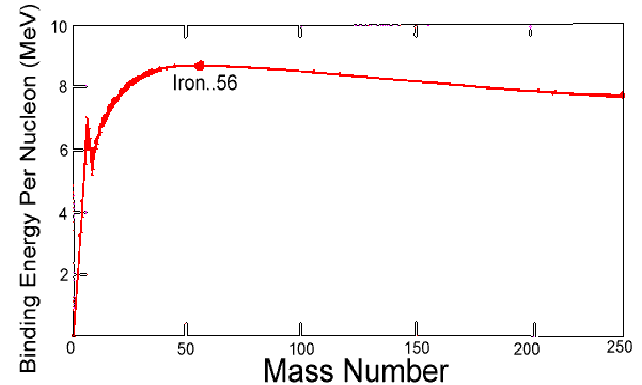
The boundary between light and heavy nuclei in the sense described above is iron. In order to make meaningful comparisons between different nuclei, the binding energy per nucleon is calculated. Then the binding energy per nucleon tends to increase with nucleon number for light nuclei and decrease with nucleon number for heavy nuclei, as in the diagram below. The most stable nucleus is iron.