The electron diffraction experiment clearly shows the wave properties of electrons. In order for diffraction to take place, an electron wave must pass through a gap of about the same order as the wavelength of the electron. The atomic spacing in crystal is such a gap. If a beam of electrons is incident on a crystal then diffraction will take place. The experimental arrangement is shown below.
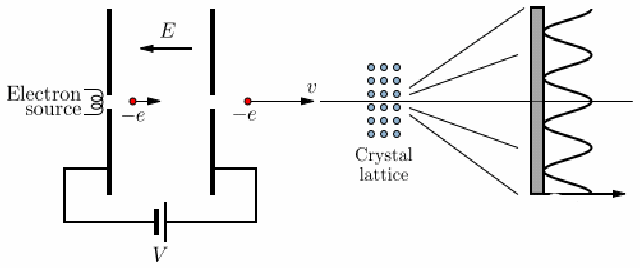
Electrons from an electron source are accelerated though a voltage V and pass through a crystal lattice, undergoing diffraction here. The diffraction pattern is displayed on a screen.
If the accelerating voltage is 1000 V, then the electrons will gain kinetic energy equal to![]()
We can find the speed of the electrons using (for non relativistic speeds)![]()
The de Broglie relationship then gives the wavelength of the electrons:![]()
The electron waves will display constructive interference in certain directions and destructive interference in others.
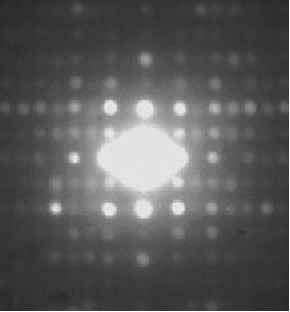
If the crystal is rotated, circles will form.
