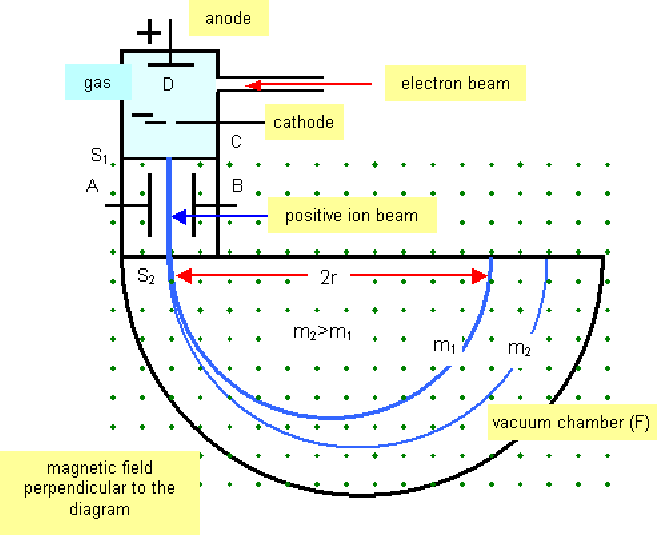
Isotopes of the same element have the same chemical properties so cannot be seaparated using chemical reactions. The mass spectrometer provides a way determining the mass of individual nuclei and separating the isotopes. A mass spectrometer is shown schematically below.
If the magnetic field has strength![]() and the speed of the isotope with charge
and the speed of the isotope with charge![]() is
is![]() then the magnetic force is of magnitude
then the magnetic force is of magnitude![]() and is at right angles to the velocity of the particle always, so forces the particle to move in a circle.
and is at right angles to the velocity of the particle always, so forces the particle to move in a circle.
The centripetal force – the force acting towards the centre of the circle – is![]() Equating the magnetic to the centripetal force gives
Equating the magnetic to the centripetal force gives![]()
Rearrangement of this gives![]()
If the ions have the same charge![]() and are selected to be travelling with the same speed, then the radius of the circle will be proportional to the mass. The mass spectrometer can thus easily find the masses of different isotopes.
and are selected to be travelling with the same speed, then the radius of the circle will be proportional to the mass. The mass spectrometer can thus easily find the masses of different isotopes.
