For a long time it was argued over whether light had a wave or particle nature. Thomas Young seemed to have settled the argument in favour of the wave nature of light, until observations of the photoelectric effect showed conclusively that light also had particle properties.
Light exists simultaneously as a wave, with wave properties – wavelength, frequency, able to undergo reflection, interference, diffraction – and particle properties – localisation, having momentum and capable of undergoing collisions with other particles, obeying the principles of conservation of momentum and energy.
De Broglie's contribution to quantum physics was to generalise the light model as a wave particle synthesis to all particles. If light, which most people supposed to be a wave, could exhibit particle properties, then maybe matter particles could exhibit wave properties. The relationship between the wave and matter properties of particles and light appears in the form of an equation
![]()
where![]() = the momentum of the particle (or light photon)
= the momentum of the particle (or light photon)
![]() = Planck's constant
= Planck's constant
![]() = wavelength
= wavelength
In fact all particles and waves exist as wavepackets that label the position of the particle.
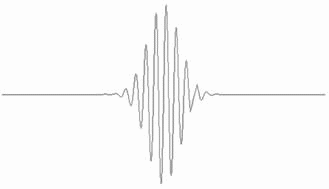
The waves that constitute the wavepacket interfere constructively towards the centre of the wavepacket but less so towards the edge, and exhibit complete destructive interference outside the wavepacket.
Wave particle duality generalises to ordinary things – tables chairs etc -but the momentum of these ordinary things is so large that the wavelength is very small and we think of them as ordinary matter.
