The nature of the atom was unknown until the early twentieth century but various models existed. One of the best contenders was of the atom as a plum pudding, the atom as a whole being a plum pudding, pitted throughout with electrons. All atoms were thought to contain electrons. These were thought to be much smaller than an atom and had been shown to be negative. Since an atom had to be neutral overall, any atom had to include an equal amount of positive charge to balance the negative charge on the electrons.
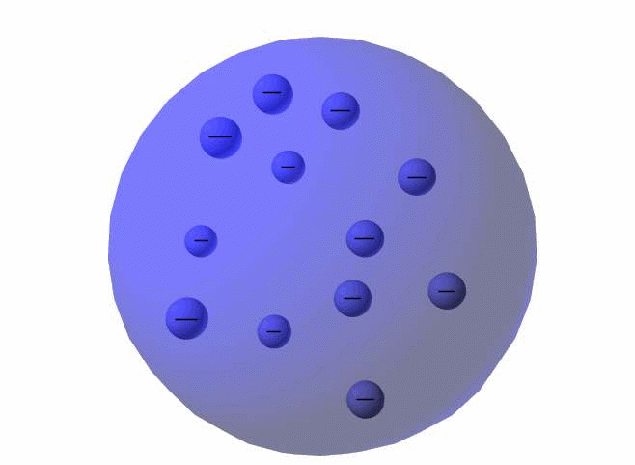
The electrons were held together in an atom by a positive 'glue', fixed in place in the same way that plums in a plum pudding were held together by glue.
The plum pudding model was a reasonable working model. The pudding had a density equal to the overall density of the material, and fitted well into Newtonian mechanics which pictured atoms as billiard balls. It was also not inconsistent with phenomena such as the photoelectric effect – energy would be needed to extract the electrons from the glue that held them in place. It could possibly be reconciled with the periodic table too, with had electrons arranged into shells, by arranging electrons periodically within the pudding.
