The particle moves fastest in regions of minimum potential energy. Since it moves fastest and has most energy there, it's wavelength is shorter. It spends least time in those regions and so the probability of finding the particle there is low, hence![]() is small. It moves slower in regions of high potential energy and spends most time in these regions, hence
is small. It moves slower in regions of high potential energy and spends most time in these regions, hence![]() is bigger there. In these regions it has less energy and it's wavelength is larger.
is bigger there. In these regions it has less energy and it's wavelength is larger.
The number of radial nodes increases by one with each increase in energy level.
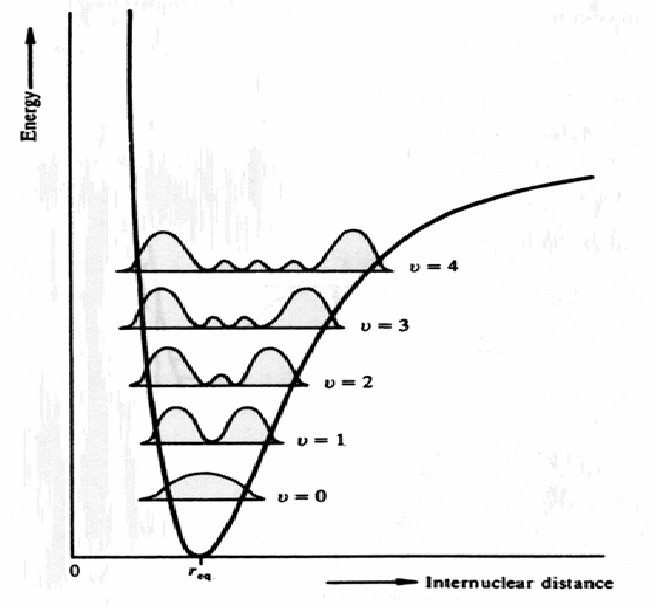
The wavefinction decreases to zero outside the quantum well since it is a bound wavefunction.
