Even after Rutherford's model of the atom as a minature solar type system had triumphed, with a heavy positively charged nucleus orbited by electrons, it was known that the posiitive particle – the proton – inside the nucleus could not be the only type of particle there. At the very least, the nuclus was too heavy. The proton did not have sufficient mass to account for the mass of an atom.
James Chadwick discovered the neutron in 1932 and measured it's mass. Bothe and Becker had already observed that when alpha particles bombarded beryllium, an uncharged radiation was emitted. Cure – Joliot and Frederic – Joliot saw that when this radiation bombarded a hydrogen containing material, for example, paraffin wax, protons were produced.
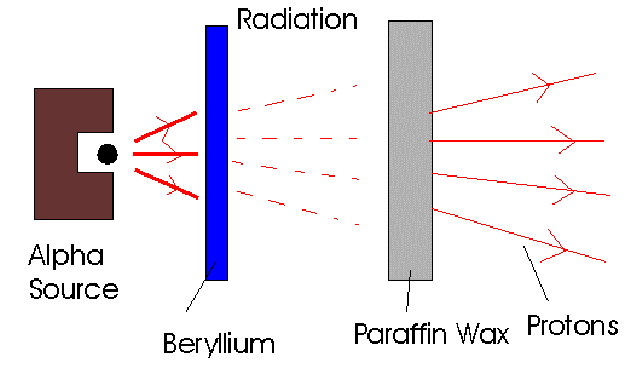
They assumed it was gamma radiation, but Chadwick showed, using the laws of conservation of energy and momentum, that the uncharged radiation must consist of particles of mass 1 and charge 0. He called them neutrons, meaning neutral.
The original alpha particle – beryllium reaction must have been
![]()
