An experiment which involved firing alpha particles at a piece of thin gold filed lead Ernest Rutherford to draw up a new model of the atom as a very small heavy nucleus surrounded by orbiting electrons.
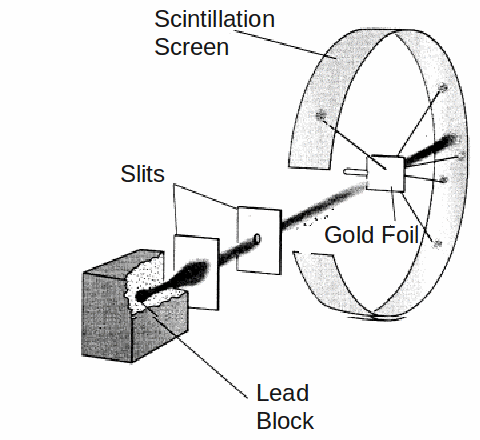
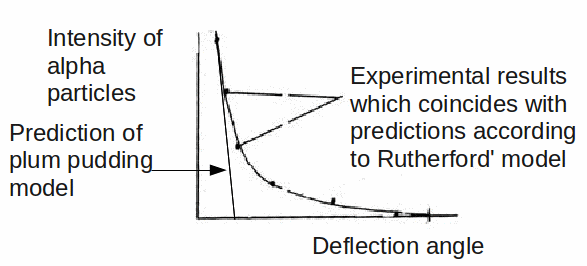
To the great surprise of Rutherford, and the two students. Geiger and Marsden, who conducted the experiment, a small but statistically signicant fract of the alpha particles were deflected though very large angles, and even more surprising, some were bounced back towards the emitter.
Thomson's plum pudding model predicted quite a different result, as shown below.